Publications
University of Vienna

Cationic Selenuranes – Bench-Stable Sources of Se(III) Radicals
Zhiliaev, K., Maryasin, B., Kählig, H., Gil-Sepulcre, M., Mateos, J.*
Angew. Chem. Int. Ed., 2025, e202513534
Prior to the University of Vienna
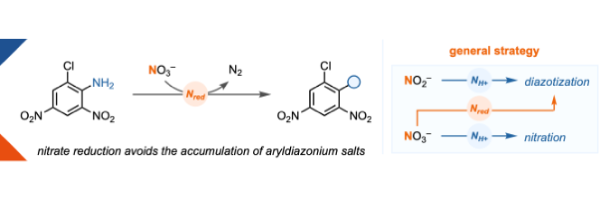
Iron-mediated nitrate reduction at ambient temperature for deaminative sulfonylation and fluorination of anilines
Schulte, T., † Behera, D.,† Carboni, D., Höppner, A., Waldbach, F., Mateos, J., Altun, A., Leutzsch, M., Krebs, M. L., F., Ritter, T.*
J. Am. Chem. Soc., 2025, 2025, 147, 16901–16908
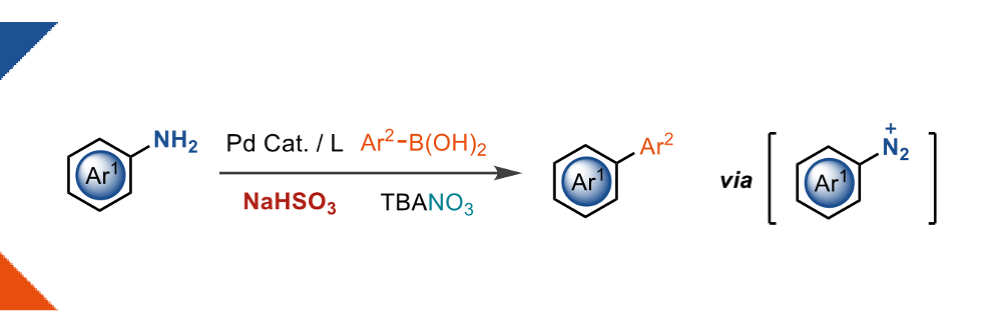
Nitrate reduction enables safer aryldiazonium chemistry
Mateos, J.,† Schulte, T., † Behera, D., Leutzsch, M., Altun, A., Sato, T. Schnegg, A., Neese, F., Ritter, T.*
Science, 2024, 384, 446–452
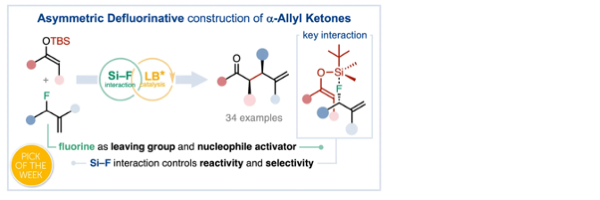
Catalytic asymmetric defluorinative allylation of silyl enol ethers
Duran, J., Mateos, J., Moyano, A., Companyó, X.*
Chem. Sci., 2023, 14, 7147–7153
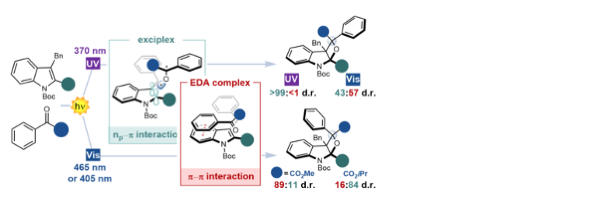
Unveiling the impact of the light source and steric factors on [2+2]-heterocycloaddition reactions
Mateos, J., Rigodanza, F., Costa, P., Natali, M., Vega-Peñaloza, Fresch, E., Collini,E., A., Bonchio, M., Sartorel, A., Dell’Amico, L.*
Nat. Synth., 2023, 2, 26–36
Halloysite nanotubes as bimodal Lewis/Brønsted acid heterogeneous catalysts for the synthesis of heterocyclic compounds.
Yu, J., Mateos, J., Carraro, M.*
Nanomaterials, 2023, 13, 394
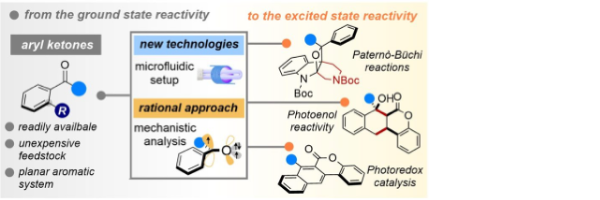
Unlocking the synthetic potential of light-excited aryl ketones: applications in direct photochemistry and photoredox catalysis
Mateos, J., Cuadros, S., Vega-Peñaloza, A., Dell’Amico, L.*
SynLett, 2021, 33, 116–128
High site selectivity in electrophilic aromatic substitutions: mechanism of C–H thianthrenation
Julia, F., † Shao, Q., † Duan, M., Plutschack, M., Berger, F., Mateos, J., Lu, C., Xue, X-S., Houk, K.N.,* Ritter, T.*
J. Am. Chem. Soc., 2021, 143, 16041–16054
Radical α-trifluoromethoxylation of ketones under batch and flow conditions by means of organic photoredox catalysis
Duhail, T.,† Bortolato, T.,† Mateos, J., Anselmi, E., Jelier, B., Togni, A., Magnier, E.,* Dagousset, G.,* Dell’Amico, L.*
Org. Lett., 2021, 23, 7088–7093
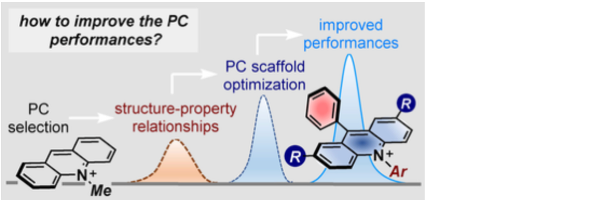
A rational approach to organo-photocatalysis. novel designs and structure-property-relationships
Vega-Peñaloza, A., Mateos, J., Companyó, X., Escudero-Casao, M., Dell’Amico, L.*
Angew. Chem. Int. Ed., 2021, 3, 1082–1097
Microfluidic visible-light Paternò-Büchi reaction of oxindole enol ethers
Franceschi, P., Mateos, J., Vega-Peñaloza, A.*, Dell’Amico, L.*
Eur. J. Org. Chem., 2020, 43, 6718–6722
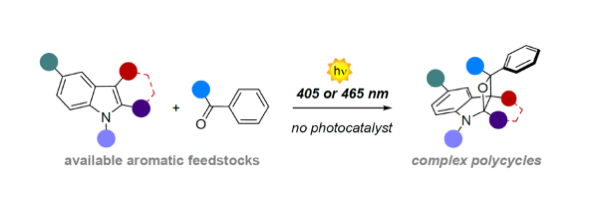
A visible-light Paternò-Büchi dearomatisation-process towards the construction of oxetoindolinic polycycles
Mateos, J.,† Vega-Peñaloza, A.,† Franceschi, P.,† Rigodanza, F., Andreetta, P., Companyó, X.*, Pelosi, G., Bonchio, M., Dell’Amico, L.*
Chem. Sci., 2020, 11, 6532–6538
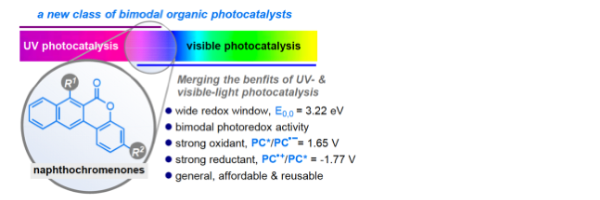
Naphthochromenones: organic bimodal photocatalysts engaging in both oxidative and reductive quenching processes
Mateos, J.,† Rigodanza, F.,† Vega-Peñaloza, A., Sartorel, A., Natali, M., Bortolato, T., Pelosi, G., Companyó, X., Bonchio, M., Dell’Amico, L.*
Angew. Chem. Int. Ed., 2020, 41, 1302–1312

Microfluidic light-driven synthesis of tetracyclic molecular architectures
Mateos, J., Meneghini, N., Bonchio, M., Marino, N., Carofiglio, T., Companyó, X.*, Dell’Amico, L.*
Beilstein J. Org. Chem., 2018, 14, 2418–2424
Transition metal-free CO2 fixation into new carbon-carbon bonds
Cherubini-Celli, A., Mateos, J., Bonchio, M.*, Dell’Amico, L.*, Companyó, X.*
ChemSusChem, 2018, 11, 3056–3070
Luminescent supramolecular heterometallic macrocycles and their encapsulation on cholate gels
Gavara, R.*, Mateos, J., Sabaté, F., Belda, R., Llinares, J. M., García-España, E.*, Rodríguez, L.*
Eur. J. Inorg. Chem., 2018, 4550–4555
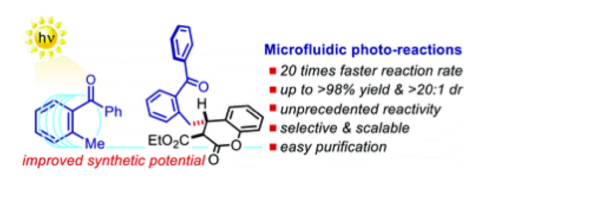
A microfluidic photoreactor enables 2-methylbenzophenone light-driven reactions with superior performance
Mateos, J., Cherubini-Celli, A., Carofiglio, T., Bonchio, M., Marino, N., Companyó, X.*, Dell’Amico, L.*
Chem. Commun., 2018, 54, 6820–6823